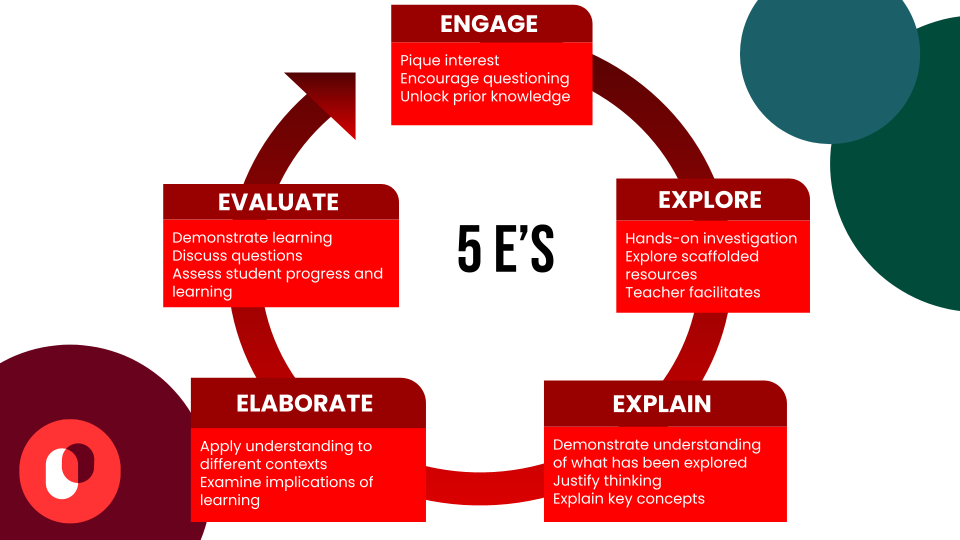
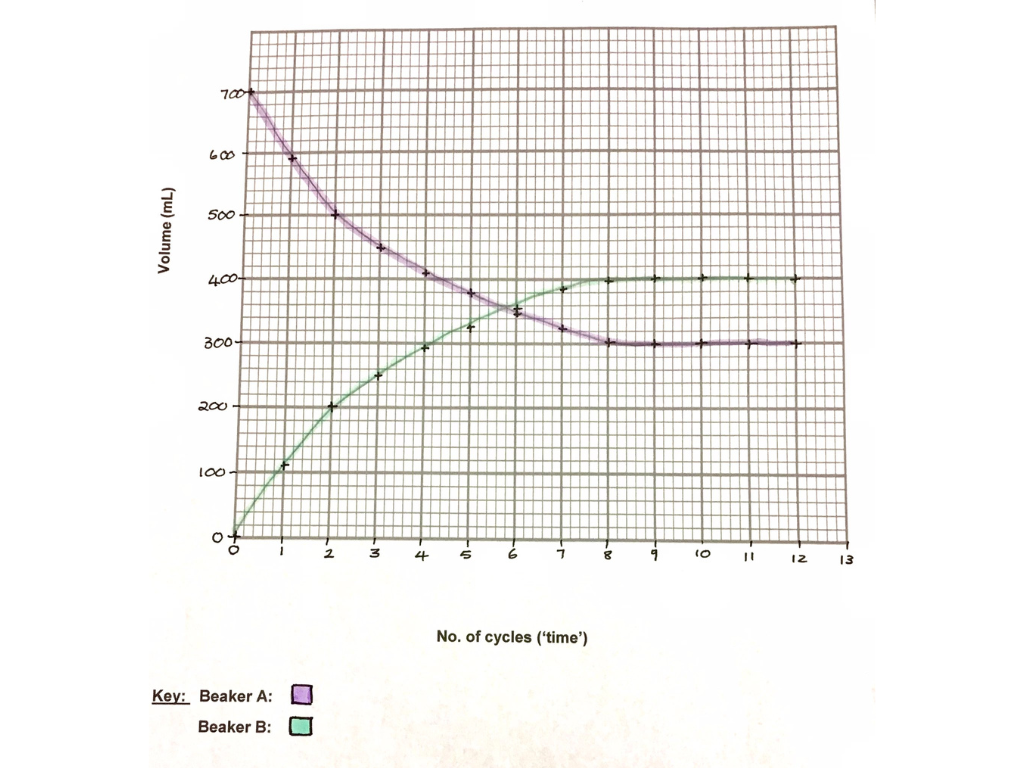
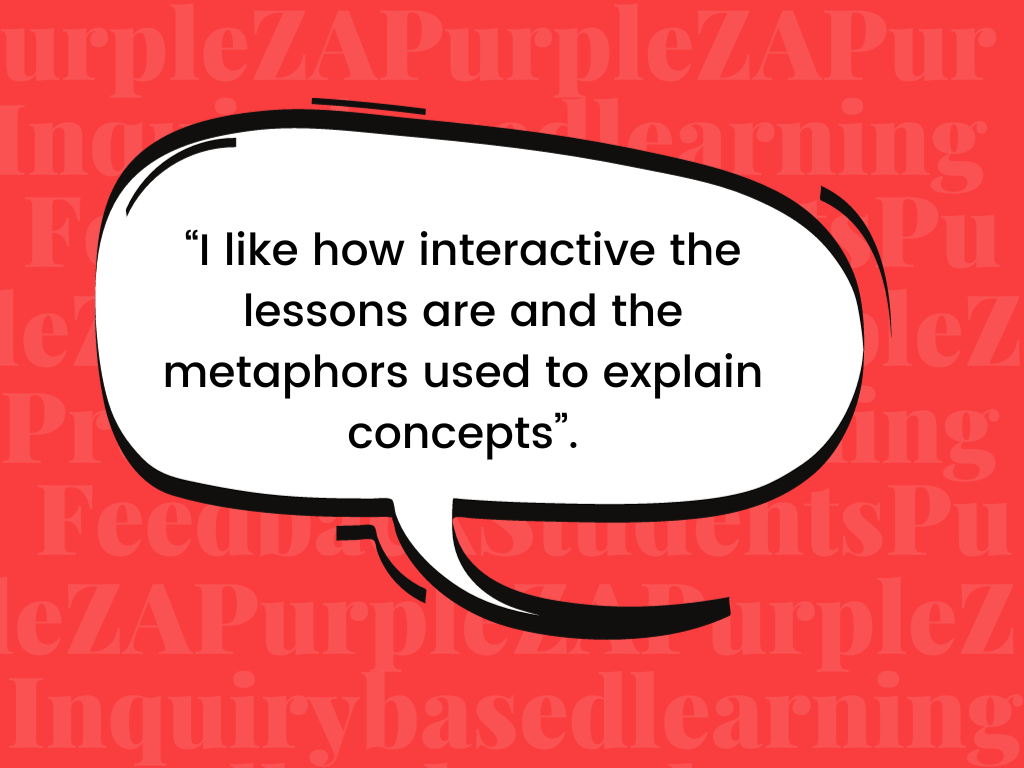
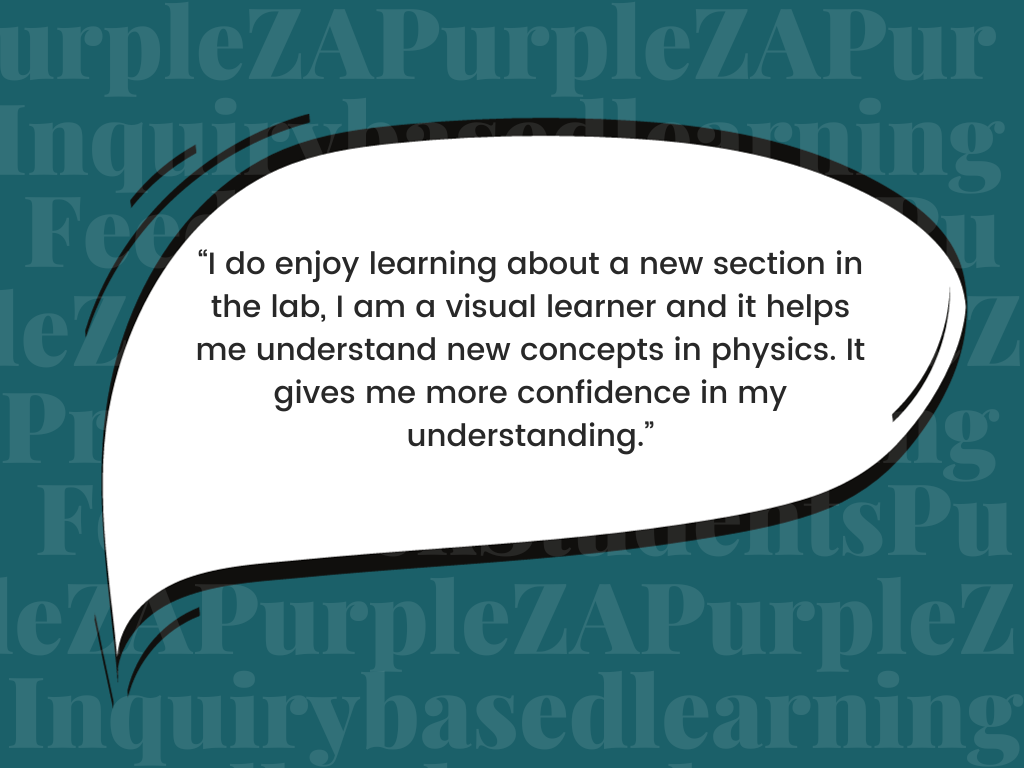
The St. Mary’s Physical Science department has been intentional over the years in trying to use the Inquiry-Based Learning approach more, and in bringing CURIOSITY back to our Science classrooms. We believe that science teaching should be driven by student inquiry – when students take ownership of their learning when they play a role in the learning process and the construction of their knowledge, confidence will grow.